Research Interests
We study how individual cells acquire and change identity, shape and function and thereby build and maintain functional tissues and organs during development and throughout adult life. We use genetic/genomic, cellular/molecular, imaging and computational approaches to investigate these developmental processes in Drosophila.
• Molecular Control of Cell Fate Decisions
During development, cells often adopt one of two possible alternative fates. Binary fate choice often involves cell-cell communications that are mediated by the receptor Notch and its ligand Delta. A main focus of our research is therefore concerned with the regulation of Delta-Notch signalling. To study regulation of Notch in vivo, we are developing imaging tools to monitor in real time the activity of Notch. We have recently obtained a fully functional Notch-GFP receptor that can be used to monitor signalling activity, i.e. nuclear Notch, with single cell resolution using in vivo GFP live imaging. Additional probes are being designed to study the regulated trafficking of Notch and Delta in vivo.
We study the developmental regulation of Notch activity in two main contexts. First, we study the dynamics of Notch-mediated lateral inhibition in developing pupae. Adult sensory organ precursor cells (SOPs) are singled out from groups of equipotent cells endowed with the potential to become SOPs by proneural transcription factors. We investigate how proneural factors cross talk with Notch to generate the regular pattern of SOPs in this tissue.
Second, we study the molecular control of asymmetric cell division. Once specified, each SOP follows a stereotyped series of asymmetric divisions. The first division takes place within the plane of the epithelium and along the body axis to generate an anterior pIIb cell and a posterior pIIa cell (the orientation of this division is regulated by the Planar Cell Polarity). Two regulators of Notch signalling are unequally segregated into pIIb and act in parallel to confer directionality to Notch signalling between pIIa and pIIb. The E3 ubiquitin ligase Neuralized targets the Notch ligand Delta for endocytosis and thereby promotes Notch receptor activation in pIIa. The endocytic adaptor Numb acts at cytokinesis to create an asymmetry of Notch, thereby inhibiting Notch in pIIb. We study how Numb, Neuralized and other factors act at the molecular level to set directional Notch signalling during asymmetric cell division.
We also perform molecular and genetic screens for modulators of Notch signalling. We are currently characterizing the function of several putative regulators of Notch. In particular, our recent work has indicated that complex glycosphingolipids positively regulate the trafficking and signalling activities of the Notch ligands.
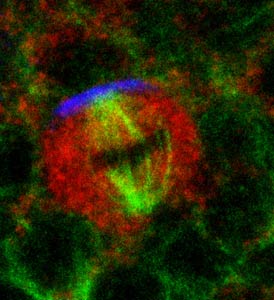
Numb (blue) localizes at the anterior pole of the pI cell. the mitotic spindle (green) lines up with Numb
• Transcriptional Programs and Morphogenesis
A second aspect of our work aims at understanding how cell shape is developmentally regulated. We focus on the remodelling of epithelial polarity. In the embryo, we study the apical constriction of the ventral cells and the invagination of the mesoderm during gastrulation. We also study the remodelling of the midgut epithelium that facilitates the trans-epithelial migration of the germline pole cells across this epithelium. In these two settings, we study the potential role of the E3 ubiquitin ligase Neuralized and of its inhibitors of the Bearded family. We also investigate the remodelling of epithelial polarity in SOPs that precedes their asymmetric cell division in the pupal notum. Using live imaging, we study how planar asymmetry arises prior to mitosis. Here, a main issue is to decipher how acquisition of the SOP fate leads these cells to divide in an asymmetric manner.
Linking transcriptional programs to changes in epithelial polarity is a key issue in morphogenesis. Since transcription is a primary read-out of genomic information, extracting cis-regulatory information from genomic data is one possible approach to investigate the genetic program active in SOPs. In collaboration with Vincent HAKIM (ENS, Paris), we have developed tools for the genome-wide identification of cis-regulatory motifs and modules underlying gene co-regulation using statistics and phylogeny. These tools have been applied to the SOP-specific regulatory sequences and have led to the identification of Drosophila genes that are upregulated in SOPs. We are currently combining this in silico approach with transcriptomic (including RNAseq of laser-captured SOPs) and transcription factor binding data (using cell-specific DamID) to further characterize the SOP genetic program and its functional relevance for SOP-specific polarity remodelling.
• Stem Cells and Tissue Homeostasis
A further aspect of our work concerns the maintenance, self-renewal and/or differentiation of multipotent progenitor cells. Notch signalling has recently been shown to regulate tissue homeostasis in the adult gut by regulating self-renewal and differentiation of adult intestinal stem cell (ISC) lineage. ISCs divide to produce one self-renewed ISC and one post-mitotic cell that becomes an absorptive enterocyte or a secretory enteroendocrine cell. We have recently shown that different levels of Notch signalling are implicated in commitment and differentiation of ISCs in adult flies. How Notch signalling is modulated over time and whether temporal fluctuations matter for tissue homeostasis remain to be addressed.